Clinical Trial Begins for Device to Treat Spasticity in Cerebral Palsy, Other Disorders

An Institutional Review Board (IRB)-approved human clinical trial has been launched by Boston-based PathMaker  Neurosystems to evaluate the safety and efficacy of the company’s MyoRegulator PM-2200 medical device in treating spasticity in neuromotor disorders, including cerebral palsy.
Neurosystems to evaluate the safety and efficacy of the company’s MyoRegulator PM-2200 medical device in treating spasticity in neuromotor disorders, including cerebral palsy.
PathMaker’s development project is being conducted in collaboration with Northwell Health — New York State’s largest healthcare provider with 21 hospitals and nearly 450 outpatient practices, serving more than 1.8 million people annually in the metro New York area and beyond, and Northwell Health’s research arm, The Feinstein Institute for Medical Research headquartered in Manhasset, New York.
 MyoRegulator PM-2200 is PathMaker’s first product, and the hardware platform for the trial subject’s objective — development of a non-invasive neurotherapy system to treat neuromotor disorders including spasticity, a common condition afflicting many patients suffering from cerebral palsy, stroke, multiple sclerosis, spinal cord injury, and traumatic brain injury. Spasticity is characterized by tight or stiff muscles, and the inability to exercise control of those muscles. More than 15 million patients worldwide are estimated to be affected by spasticity.
MyoRegulator PM-2200 is PathMaker’s first product, and the hardware platform for the trial subject’s objective — development of a non-invasive neurotherapy system to treat neuromotor disorders including spasticity, a common condition afflicting many patients suffering from cerebral palsy, stroke, multiple sclerosis, spinal cord injury, and traumatic brain injury. Spasticity is characterized by tight or stiff muscles, and the inability to exercise control of those muscles. More than 15 million patients worldwide are estimated to be affected by spasticity.
PathMaker says in a press release that spasticity management is currently difficult and sometimes proves an insurmountable challenge that confounds currently available pharmacological, surgical, and physical treatments which have, at best, short-term efficacy with frequent unpleasant side-effects. As a result, the company perceives a tremendous unmet medical need for more effective spasticity treatments.
Patients with spasticity have damaged neural pathways that give rise to hyperexcitable spinal circuits. The MyoRegulator device is based on PathMaker’s proprietary DoubleStim technology, which provides simultaneous non-invasive stimulation at spinal and peripheral sites via a technique called trans-spinal Direct Current Stimulation (tsDCS) to suppress these hyperexcitable spinal circuits in spasticity patients. PathMaker says DoubleStim technology has been shown in published animal studies to be capable of suppressing hyperexcitability, thereby leading to reduced muscle spasticity.
Last October, the MyoRegulator PM-2200 system received Expedited Access Pathway (EAP) designation and priority processing status from the U.S. FDA, under a new FDA program to help patients gain more rapid access to critical medical devices by expediting a product’s development, assessment, and review. The EAP designation is due to MyoRegulator’s intended use in treating irreversibly debilitating conditions, and because it offers significant, clinically meaningful advantages over existing alternatives.
“The EAP designation for MyoRegulator is significant, as FDA reserves this priority review program only for devices with demonstrated potential to fill an unmet medical need and benefit patient health,” said attorney Sheila Hemeon-Heyer, vice president of Regulatory and Clinical Affairs at PathMaker Neurosystems. “We are excited to be working with FDA through the EAP program to rapidly bring to patients this important new technology for treating spasticity.”
“Through the FDA Expedited Access Pathway program, we will be able to more rapidly bring to market this breakthrough technology for treating muscle tone disorders,” said Nader Yaghoubi, MD, Ph.D. , president and CEO of PathMaker. “As one of the first companies selected into this program, we look forward to working with FDA to rapidly make MyoRegulator available to patients and clinical institutions worldwide.”
PathMaker also announced that it has received positive FDA confirmation that clinical trials for MyoRegulator constitute non-significant risk (NSR) device studies. NSR designation by the FDA allows clinical trials to proceed in the U.S. without the need to submit an Investigational Device Exemption (IDE) application, when an FDA assessment determines they are not considered to present potential for serious risk to the health, safety, or welfare of a subject.
The company says clinical use of DoubleStim technology has demonstrated particularly encouraging results treating spasticity related to cerebral palsy. IRB-approved human clinical trials are now underway to support regulatory submissions for the MyoRegulator system.
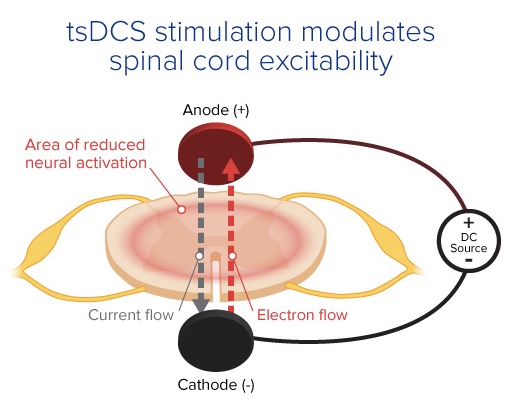
The cross-sectional illustration below depicts placement of electrodes in relation to the spinal cord in tsDCS. The anode (shown in red) is positioned dorsally and placed on the skin covering the spinal cord at the desired spinal level, while the cathode (shown in black) is positioned ventrally on the abdomen. As current flows from anode to cathode, an area of reduced neural activation is created within the spinal cord, and by reversing the polarity of the electrodes, an area of increased neural activation can be achieved.
Image courtesy PathMaker Neurosystems
Depending on the stimulations and polarities induced, tsDCS can induce areas of increased or decreased neural activation within the spinal cord, allowing clinicians to bi-directionally modulate spinal cord excitability. This effect, combined with neurostimulation at peripheral and cortical sites, enables modulation of physiology beyond what is possible with single-site stimulation.
“Initiating our company’s first formal human clinical trial represents a significant milestone in our strategy to rapidly and efficiently bring to market an entirely new, non-invasive approach to treating spasticity,” Yaghoubi said in the release. “We are delighted to be working with Northwell Health and The Feinstein Institute for Medical Research, leaders in the emerging field of bioelectronic medicine.”
Bioelectronic medicine is a scientific discipline that melds elements of molecular biology, neurophysiology, neurotechnology, and analytics to develop nerve-stimulating technologies capable of regulating molecular targets underlying disease — an approach promising to enable the development of therapies superior to pharmaceuticals in terms of efficacy, safety, and cost, and without significant side effects.
At the core of bioelectronic medicine, which has been researched and developed for nearly 20 years by investigators at the Feinstein Institute, are the electrical signals used by the nervous system to communicate information with virtually every cell of the body, directly or indirectly, controlled by these neural signals. Bioelectronic medicine technologies can record, stimulate, and block neural signaling, and they will change the way diseases, injuries, and conditions such as rheumatoid arthritis, Crohn’s disease, diabetes, paralysis, bleeding, and even cancer are treated.
 “We are thrilled to partner with PathMaker and begin studying a new treatment that could provide relief for patients who suffer from spasticity, a serious condition that can often inhibit patients from participating in everyday activities,” said Bruce T. Volpe, M.D., lead investigator of the study and investigator of the Laboratory of Biomedical Science at the Feinstein Institute. “I see patients who can no longer open doors or feed themselves as a result of suffering from spasticity. It is my hope that at the conclusion of this trial, we will see that MyoRegulator is a safe and effective treatment option.”
“We are thrilled to partner with PathMaker and begin studying a new treatment that could provide relief for patients who suffer from spasticity, a serious condition that can often inhibit patients from participating in everyday activities,” said Bruce T. Volpe, M.D., lead investigator of the study and investigator of the Laboratory of Biomedical Science at the Feinstein Institute. “I see patients who can no longer open doors or feed themselves as a result of suffering from spasticity. It is my hope that at the conclusion of this trial, we will see that MyoRegulator is a safe and effective treatment option.”
PathMaker was a presenter at the 11th Annual Neurotech Investing and Partnering Conference earlier this month in Boston, in the Emerging Company Showcase. The conference is the premier partnering and investing event for the neurotechnology industry, including pharmaceuticals, medical devices, software, and diagnostics for the brain and nervous system. It is a global forum where investors, executives, entrepreneurs, scientists, and others involved in the development of new treatments and diagnostics for the brain and nervous system come together to shape the future of their organization and the industry.
With offices in Boston, Massachusetts, and Paris, France, PathMaker has a trans-Atlantic strategy in place to rapidly bring to the market its approaches to non-invasively treat neuromotor disorders. The company notes that 48 million patients in the U.S., Europe, and China suffer disabilities due to cerebral palsy, stroke, multiple sclerosis, spinal cord injury, traumatic brain injury, Parkinson’s disease, and other neurological disorders.